Endelig en studie som viser direkte hva som skjer med CO2 når vi holder pusten etter utpust. Her kaller de det etter «functional residual capacity» (wikipedia), altså etter en normal og passiv utpust. Denne studien viser først og fremst hva som skjer i løpet av én enkelt pustehold. De nevner også at det tar 5-10 sekunder etter innpust igjen før CO2 nivet begynner å synke. Så i metablsk pust (RecoveryBreathing) hvor vi puster 10sek inn/ut og har 10 sek pause bør CO2 lett kunne stabiliseres på et høyere nivå enn normalt.
http://www.ncbi.nlm.nih.gov/pubmed/8874252
Hele Studien: http://journal.publications.chestnet.org/data/Journals/CHEST/21737/958.pdf
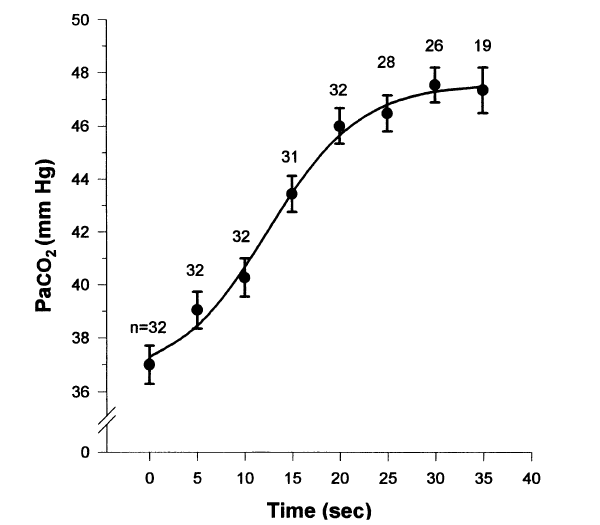
Breath-holding serves as a model for studying gas exchange during clinical situations in which cessation of ventilation occurs. We chose to examine the arterial blood gas changes that occurred during breath-holding, when breath-holding was initiated from functional residual capacity (FRC) while breathing room air. Eight normal subjects who had a radial artery catheter placed for another study were taught to breath-hold on command from FRC. FRC was determined using respiratory inductance plethysmography. Arterial blood gas specimens were obtained at 5-s intervals until the termination of breath-holding. The average breath-holding time (+/-SD) was 35 (+/-10 s). The PaO2, PaCO2, and pH values were plotted against time and individually fit to logistic equations for each subject. The arterial PaO2 fell by a mean of 50 mm Hg during the first 35 s of breath-holding under these conditions, while the arterial PCO2 rose by a mean of 10.2 mm Hg during the first 35 s and the pH fell by a mean of 0.07 in the first 35 s. The rapid decline in PaO2 is greater than that previously reported using different methods and should be considered in clinical situations in which there is an interruption of oxygenation and ventilation at FRC while breathing room air. The changes in PaCO2 and pH are similar to those previously reported in paralyzed apneic patients.
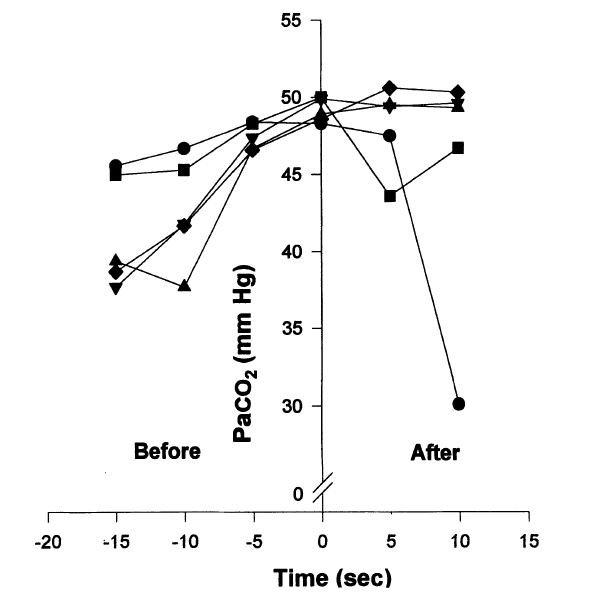
We demonstrated during 5 breath-holding runs in which additional arterial specimens were obtained at 5 and 10 s after breath-hold (Fig 6) that the elevated arterial PCO2 did not begin to fall until at least 5 s after breaking from the breath-hold in 1 run and greater than 10 s in 3 other runs. This implies that the removal of the remaining arterial PCO2 by the lungs took longer than 5 s before recirculation from pulmonary capillary blood could lower the arterial PCO2 in the radial artery. The second less significant factor that explains the persistent elevation of arterial PCO2 is the concentrating effect caused by the decreasing LV. The concentrating effect occurs with breath-holding, as more oxygen is removed from the lungs than carbon dioxide is added. As carbon dioxide production continues to occur, the capillary